Introduction
Expansion microscopy is a new technique in microscopy which uses special expanding
gels to
facilitate observation of microscopic objects. The basic technique is that, we add
the object of interest
(the cell for example) in these gels. These gels have a property that on addition of
water they expand.
The expansion on the gel makes the cell of interest also expand. Thus we can make
the cells and its constituents
bigger without denaturing them. For example, initially the cell size was 1X after
the expansion in the gel
it will be 10-20X thus we are automatically getting a magnification of 10 to 20x
without any costly apparatus.
Though the expansion gel can be made to expand 100s of folds, that might not be the
case with the cell.Traditional light microscopy has limits of resolution that
prevent it from reliably distinguishing small biological structures, and must
instead be imaged by a higher-resolution technique, such as electron microscopy.
ExM offers a cheap alternative to this.
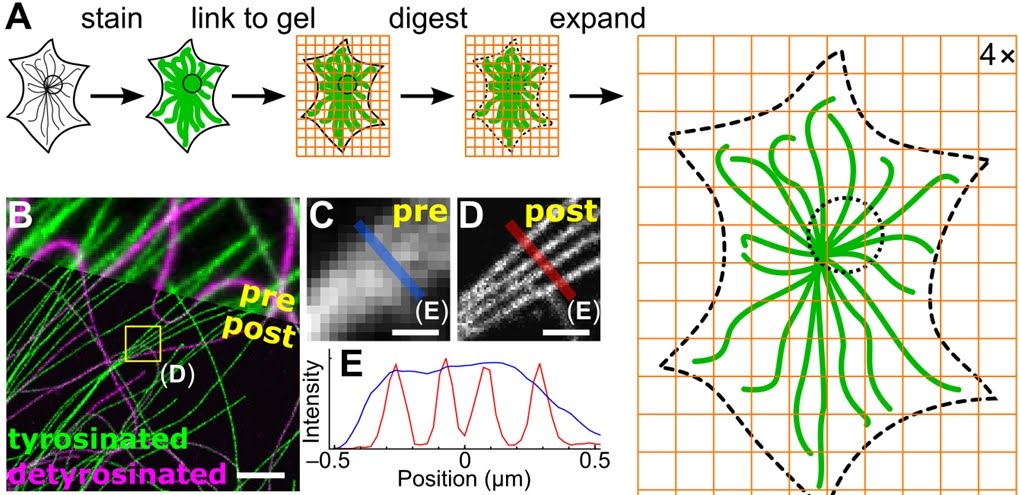
There
might be a threshold expansion beyond the which the proteins will break and the
cell organelles will get damaged.
Thus its critical to understand this threshold for using expansion microscopy.
The commonly used gel contains polyacryl amid, which expands on addition of water.
This material is a common constituent
of diapers.
This is a interesting ted talk on expansion microscopy, specially on using this technique for uncovering the secrets of the brain. The following is a gelation demonstration on proExM (protein retention expansion microscopy) for tissues.
Reagents Required for the homework assignments
| Reagent | Vendor | Part Number |
|---|---|---|
| Acrylamide (AA) | Sigma | A9099 |
| N,N′-Methylenebisacrylamide (BA) | Sigma | M7279 |
| Ammonium Persulfate (APS) | Sigma | A3678 |
| N,N,N′,N′-Tetramethylethylenediamine (TEMED) | Sigma | T7024 |
| Sodium chloride | Sigma | S9888 |
| 10x PBS | Sigma | D1408 |
| 1M Tris pH 8.0 | Thermo Fisher Scientific | 15568025 |
| Bovine serum albumin (BSA) | Sigma | A2153 |
| Rabit anti-E. coli polyclonal antibody | Abcam | ab137967 |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) | Abcam | ab150077 |
| Acryloyl-X | Thermo Fisher Scientific | A20770 |
| DMSO | Sigma | M81802 |
| Proteinase K | New England Biolabs | P8107S |
| Triton X-100 | Sigma | T8787 |
| EDTA | Thermo Fisher Scientific | AM9260G |
| Microscope Slides (3"x1" or similar) | VWR | 48300-026 |
| Coverslips (No. 1.5 or similar, 22mmx22mm or similar) | VWR | 48366-227 |
| Atto488 NT Labeling Kit (10 reactions) | Jena Bioscience | PP-305S-488 |
| Dextran Sulfate 50% Solution | Millipore Sigma | S4030 |
| SSC Buffer 20X Concentrate | Sigma | S6639 |
| Formamide | Sigma | F9037 |
| Very small watercolor paintbrush or similar | Utrecht Art Supply Co | 09311-1001 |
| QIAamp DNA Mini Kit 50 (or similar DNA purification kit) | Qiagen | 51304 |
| Ethanol |
Exercise 1: Cast an expanding gel
Reagents Needed
| Chemical Name | Supplier | Part Number |
|---|---|---|
| Sodium Acrylate (See Notes 1 and 2) | Sigma | 408220 |
| Acrylamide | Sigma | A9099 |
| N,N′-Methylenebisacrylamide | Sigma | M7279 |
| Ammonium Persulfate (See Note 2) | Sigma | A3678 |
| N,N,N′,N′-Tetramethylethylenediamine (see Note 2) | Sigma | T7024 |
| Sodium chloride | Sigma | S9888 |
| 10X PBS | Sigma | D1408 |
Protocol
- Step 1. Prepare the Monomer Solution (can be stored at -20 for at least a
month):
Chemical Name Supplier Part Number Sodium Acrylate (See Notes 1 and 2) Sigma 408220 Acrylamide Sigma A9099 N,N′-Methylenebisacrylamide Sigma M7279 Ammonium Persulfate (See Note 2) Sigma A3678 N,N,N′,N′-Tetramethylethylenediamine (see Note 2) Sigma T7024 Sodium chloride Sigma S9888 10X PBS Sigma D1408 - Step 2. Prepare gelation chamber: Obtain two glass slides (~ 3"x1"), two coverslips (~ 22mmx22mm), and some parafilm. Wrap one of the slides in parafilm such that one face of the slide has a smooth flat surface one parafilm layer thick. (The other face of the slide will have the folded edges of the parafilm on it.) Press the parafilm on the flat side to ensure there is no gap between the parafilm and the slide. Place a cover-slip at each end of the parafilm-wrapped slide on its smooth face. Place this parafilm-wrapped slide with coverslips into a petri dish or any container with a lid. Set the remaining glass slide aside.
- Step 3. Prepare 10% (v/v) TEMED and 10% (w/v) APS. 10% TEMED can be made by simply diluting pure TEMED tenfold in water. 10% APS can be made by measuring out some APS, finding its weight in milligrams, multiplying the weight by 9.5, and then adding that many microliters of water. Eg for 30 mg of APS, add 285 ul of water. (This assumes that 10% (w/w) APS is ~ 5% (v/w), which is approximately correct.)
- Step 4. Prepare Gelation Solution: Mix the following 4 solutions on ice: Monomer solution, 10% TEMED (accelerator), 10% APS (initiator solution). (APS initiator solution needs to be added last to prevent premature gelation). Solutions should immediately be vortexed or pipetted up and down after mixing to ensure full mixing and the gel should then immediately be cast to prevent premature gelation. For 50 µL gelling solution, mix the following: a) Monomer solution (48µl) b) Accelerator solution (1µl): 10% TEMED (TEMED stock solution at 10%, final concentration 0.2% (w/w). (Accelerates radical generation by APS). c) Initiator solution (1µl): APS (APS stock at 10%, final concentration 0.2% (w/w)). (This initiates the gelling process. This needs to be added last).
- Step 5. Polymerize the Gel: Pipet 10 ul of gelation solution onto the flat surface of the parafilm-wrapped slide between the coverslips and place the other glass slide on top so that it rests on the coverslips, leaving a small space between the two slides where the gel will polymerize. Put a wet kim-wipe or tissue in the container to keep the air in the container hydrated. Put the lid on the container and place it in a 37C incubator for 1 hour (or 1.5 hours at room temperature) to polymerize. (Note that the most ideal condition for polymerization is oxygen-free because oxygen exposure inibits the polymerization process. However, only the edges of your gel will be affected by air exposure, and the middle of the gel will polymerize well.)
- Step 6. Expand the Gel: Pry apart the two slides using foreceps, a razor blade, or some other thin edge. Put ~ 0.5 cm of deionized water in a petri dish or other container. Wet the paintbrush with water, use it to gently peel the gel off the slide and transfer it into the container with water. Let it sit in the water for ~10 mins, then remove the water and replace it with fresh water. Repeat this wash step 2-3 more times. The gel will expand ~4-fold. Be careful not to suck the gel up into a pipettor while changing the water!
- Step 7. Play around with the gel to see how to pick it up and transfer it to other containers. The gels are very delicate when expanded, so this takes some finesse. Try picking up the gel by pushing it onto a coverslip using a paintbrush. If the gel is too large for this, cut it to make it smaller.
Exercise 2: Expansion of antibody-stained E. coli
Reagents Required(In addition to the ones used in exercise )
| Reagent |
|---|
| Ethanol |
| Triton X-100 |
| Bovine Serum Albumin |
| Rabit anti-E. coli polyclonal antibody (Abcam ab137967) |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (Abcam ab150077) |
| Acryloyl-X (See Note 1) |
| DMSO |
| Proteinase K |
| 1M Tris pH 8.0 |
| EDTA |
Protocol
- Step 1. Grow E. coli overnight in liquid culture.
- Step 2. The next day, pre-chill a solution of 70% ethanol, 1X PBS at -20 degrees C. (Eg for 1 mL, 100 ul 10X PBS, 700 ul ethanol, 200 ul pure water)
- Step 3. Take 1.5 mL of mid log-phase culture and spin it down to form a pellet. This can be done using, for example, by spinning down at 7500xg for 10 mins. If possible, keep the cells cold at 4 degrees C during this step. Depending how long your cells were growing, you may need to use more of your culture to form a pellet. An ideal pellet is ~ 40 ul in volume.
- Step 4. Remove supernatant and wash the cells by resuspending in PBS and then pelleting again. If possible, keep the cells cold at 4 degrees C during this step.
- Step 5. Fix the cells by resuspending in pre-chilled 70% ethanol, 1x PBS and keeping the cells in fixation solution in the freezer for 15 minutes at -20 degrees.
- Step 6. Spin down the cells, remove the supernatant, and resuspend in PBS. Repeat this two more times, so the cells are washed 3 times.
- Step 7. Permeabilize the cells by resuspending in 1% Triton X-100, 1x PBS for 10 mins at room temperature. Then wash the cells once by pelleting, resuspending in PBS, and pelleting again.
- Step 8. Set aside half the cells to be expanded later without antibody staining and used in Exercise 3, and move to step 9 using the other half.
- Step 9. Block the cells by resuspending in 1% bovine serum albumin, 1X PBS for 15 mins. Then wash once in PBS.
- Step 10. Stain the cells with primary anti-E. coli antibody by incubating in a 1:50 dilution of Abcam ab137967 in 1x PBS, 1% bovine serum albumin overnight at 4 degrees C. Depending on the size of your pellet, you may want to discard some of the pellet to reduce the amount of antibody you use. An acceptable final volume of antibody staining solution is ~200 ul, with a ~ 10 ul pellet suspended in it.
- Step 11. Wash the cells 3 times in 1x PBS.
- Step 12. Stain the cells with secondary goat anti-rabbit antibody by incubating in a 1:200 dilution of Abcam ab150077 in 1x PBS, 1% bovine serum albumin for 4 hours at room temperature.
- tep 13. Wash the cells twice in 1x PBS.
- Step 14. Add gel-linkable moieties to the antibodies on the cells by incubating the cells in 0.1 mg/mL acryloyl-X in PBS at room temperature for at least 6 hours, up to overnight. (0.1 mg/mL acryloyl-X can be made by diluting stock acryloyl-X (10 mg/mL in DMSO) 1:100 in PBS.)
- Step 15. Wash the cells twice in PBS. During pelleting after the second wash, also pellet the cells that were set aside in step 8. Remove the supernatant from both tubes of cells and set the pellets aside to be used in step 17.
- Step 16. Prepare for casting expansion gels by prepare two gelation chambers as described in step 3 of exercise 1, and place gel monomer solution (prepared in step 1 of exercise 1) and 10% APS and 10% TEMED on ice.
- Step 17. (This step should be done as quickly as possible to avoid premature polymerization of the gel.) On ice, add 1 ul each of 10% TEMED and 10% APS to 48 ul of monomer solution and mix immediately by vortexing or aspirating repeatedly with the pipettor. Then resuspend the antibody-stained pellet in the gel solution you just made. Then use this solution to cast a gel as in step 5 in exercise 1. Repeat this also for the unstained cells, which will be used in exercise 3.
- Step 18. Digest the cells by incubating the gels in Proteinase K diluted 1:100
(8 units/mL) in digestion buffer (recipe below) overnight at room temperature or
at 37 degrees C for 4 hours. This can be done in a 30 mm petri dish or other
small container with a flat bottom, or in a microfuge tube. (If you plan to
image using a conventional fluorescent microscope, a 6-well glass-bottom dishes
is an excellent container in which to digest and expand gels.) The volume of
digestion buffer should be at least 100-fold the volume of the gel. If your gel
is too large for this, cut it with a razor or other sharp edge to make it
smaller. Gels can be transferred to digestion buffer using a paintbrush, similar
to step 6 in exercise 1.
Digestion Buffer:
Component Aqueous stock concentration Amount (uL) Final concentration Tris pH 8.0 1M 500 50 mM Triton X-100 10% 500 0.5% EDTA 500 mM 20 1 mM Sodium chloride 5 M 1000 0.5 M Water 7980 Total 10000 - Step 19. Expand the cells by washing the gels in deionized water 4 times for 10 minutes each, as in step 6 from exercise 1. This can be done in a petri dish or any container with a flat bottom. Note that the width of the chamber should be at least 4 times as wide as the original gel so there is room for it to expand. If your gel seems too large for this, you may wish to cut it to make it smaller. Also note that if you are transferring the gel from one vessel to another, you should be very gentle with it. The gels are delicate. They can be picked up most easily by pushing them onto a coverslip using a paintbrush.
- Step 20. Transfer the gel onto a thin glass surface that will be used for imaging. (The gel can be picked up most easily by pushing it onto a coverslip, as mentioned above.) If using the mini microscope, this can be a coverslip. If you'll be using a conventional fluorescent microscope, use a coverslip or any other vessel with coverslip-thickness glass on the bottom that can be mounted on your microscope for imaging.
- Step 21. Image the sample using fluorescence microscopy. Alexa 488, the fluorophore on the secondary antibodies that were retained in the gel, has peak excitation at 490 nm and peak emission at 525 nm, so it can be imaged using a standard 488 imaging channel. (This is the same imaging channel used to image GFP.) A lens with 40x or greater magnification is most ideal. The bacterial cells may be distributed all throughout the thickness of the gel, or they may have settled to the bottom of the gel, depending how quickly the gel polymerized relative to the sedimentation rate of the bacteria. If you can't see any bacteria in your gel, try flipping the gel over; the cells may be on the side of the gel furthest from the objective, which may be too far from the objective to see depending on its working distance.
Ethics/ safety considerations this week
Do your activities this week raise new ethics and/or safety considerations you had not considered in week 1? Describe what activities have raised these considerations and any changes you have implemented in response.
Acrylamide and other reagents used in this experiment are hazardous in case of skin contact, eye contact, ingestion or inhalation, and are also known carcinogens. Always read and follow the Material Safety Data Sheet and usePersonal Protective Equipment and follow all necessary safety procedures!