Ethics, safety and security are essential considerations throughout (and beyond!) this class. The assignment for this week to give a strong foundation on ethics, safety and security. Here i will answer a series of questions as follows.
Q1. Risk/Safety level:What is the Safety Level of Your Lab (e.g. BSL1, BSL2, other)? Do you have different spaces with different safety levels? If so, describe which activities are done in different spaces. Include a picture of your lab.
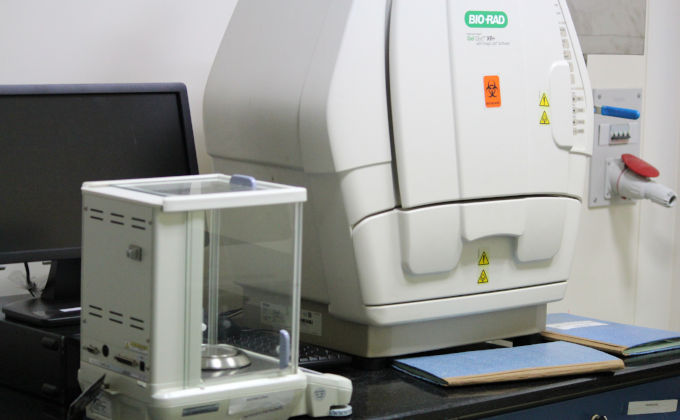
I am using the lab facility of Bionest- a biotechnology incubator and research facility in Kochi. It is a well equipped facility with many advanced machinaries. This lab has both BS1 and BS2 safety levels.
Photos of our lab : RGCB-BioNest, kochi
Q2. Work Area: Which work areas do you use for handling biological materials? (e.g open benches, biosafety cabinet, fume hoods etc)? Include a picture of your work spaces..
We have all three facilities for handling biological materials. We have open benches, biosafety cabinets as well as fume hoods.
Photos of our work area
Q3. Training: Have you received, or will you receive, any ethics and/or safety training? Who provides this training? Briefly describe any topics covered.
Extensive training on handling biochemicals, bacterial cultures, machines, were part of my graduation. Topics include the following.
- Basic safety precautions in a biotechnology lab
- Safety precautions while handling bacterial and fungal cultures
- On using laminar air flow chamber, UV related
- Autoclaving, high pressure steam related safety measures
- Ethics and biosafety
Q4. Rules and Regulations: Which laws and regulations (locally, nationally and internationally) apply to your lab? Include links to any oversight institutions/organizations and policies, and describe which specific rules are pertinent to your lab and project and why?
National Rules and Regulations
Guidelines by Indian council for Medical Research are
applicable to all the laboratories which are working in Biotechnology, Microbiology,
Molecular Biology,
Biochemistry and Clinical Diagnostics. Rules and guidelines under Environment
(Protection) Act 1986 and Rules 1989 of the Department of Biotechnology (DBT- India)
ensures safety from the use of Genetically Modified Organisms (GMOs). A three tier
mechanism comprising Institutional Biosafety Committees (IBSC) at the Institute/
company; the Review Committee on Genetic Manipulation (RCGM) in the Department of
Biotechnology; and the Genetic Engineering Approval Committee (GEAC) in the Ministry
of Environment & Forests (MoE&F) is responsible for granting approval for research
and development
activities on recombinant DNA products, environmental release of genetically
engineered (GE)crops and monitoring and evaluation of research activities involving
recombinant DNA technology. These are the regulations by
DBT India.
International Rules and Regulations
The research involving blood and body fluids in the Biosafety cabinet should adhere
to the Occupational Safety and Health administration O.S.H.A. Blood bourne
pathogen Universal standard, which is established since 1930 have to be followed for
shipping via commercial carriers,
Adherence to all DOT and IATA regulations, 49 CFR 100-185
Shipping clinical specimens need to be crosschecked with EH&S list is necessary
for our lab as most of the chemical agents and other materials are
being shipped from outside India.
Safety Regulations in our Lab
Currently we have both BSL1 and BSL2 in our lab, but is not yet fully functional. As
and when we grow and have people to use the facilities, we will keep all the safety
rules and regulations and ethical considerations in place. As of now, Safety
regulations for working with BSL2 and for the effective use of BSL2 in our lab as we
mostly work with BSC, we follow the following safety procedures.
- Make sure that BSC is certified annually
- Regularly check air flow
- Clean work area with disinfectant
- Vacuum assisted pipette aids
Personal Safety equipment
- Laboratory coat
- Shoes covering the legs
- Gloves
- Liquid resistant apron for autoclaving liquids
- Goggles to protect eyes
- Protective face masks to protect face
Q5. Organization and Practices: How do you enforce these rules? Who is responsible for ensuring safety in your lab/space? What happens when safety issues are raised?
There is a lab assistant who make sure that people entering the lab follows the rules and personal safety precautions. Also we have made measures to give a training for all those planning to work in the lab. We have also sticked precautions near each equipments. Also there is a safety commitee who takes care of enforcing and solving safety and safety issues within the institute where our lab is located.
Q6. Uncertainties: Are there any areas where you are uncertain about how to apply these rules, and whether they are relevant to your lab and/or work?
I am quite unsure about the rules when it comes to tissue engineering and related area. I plan to keep reading and reviewing these regulations as a progress with the course.