Synthetic Development Biology
Lab Assignment
Cartilage Tissue Engineering Lab
Media Making:
The following reagents were mixed to make the media:
- DMEM
- 10% FBS or 1X ITS
- 50 μg/mL ascorbic acid
- 1X essential amino acid
Collagenase Solution:
The collagenase solution was prepared by adding 0.5 mg/ml collagenase in 30ml media and then the solution was sterile filtered into 50ml tube.
Cell preparation:
- Wash intact chicken thigh quarters soapy water.
- Soak intact chicken thigh quarters in 70% ethanol for 15 minutes.
- Spray aluminum foil with ethanol and wrap thighs in foil.
- Spray outside of aluminum foil with ethanol and transfer to biosafety cabinet.
- Prepare 50 mL tubes with 25 mL PBS.
- Open up aluminum foil and remove chicken.
- Carefully cut away the flesh from the thighs.
- Open the joints.
- With a new scalpel, gently shave away the cartilage from the bone.
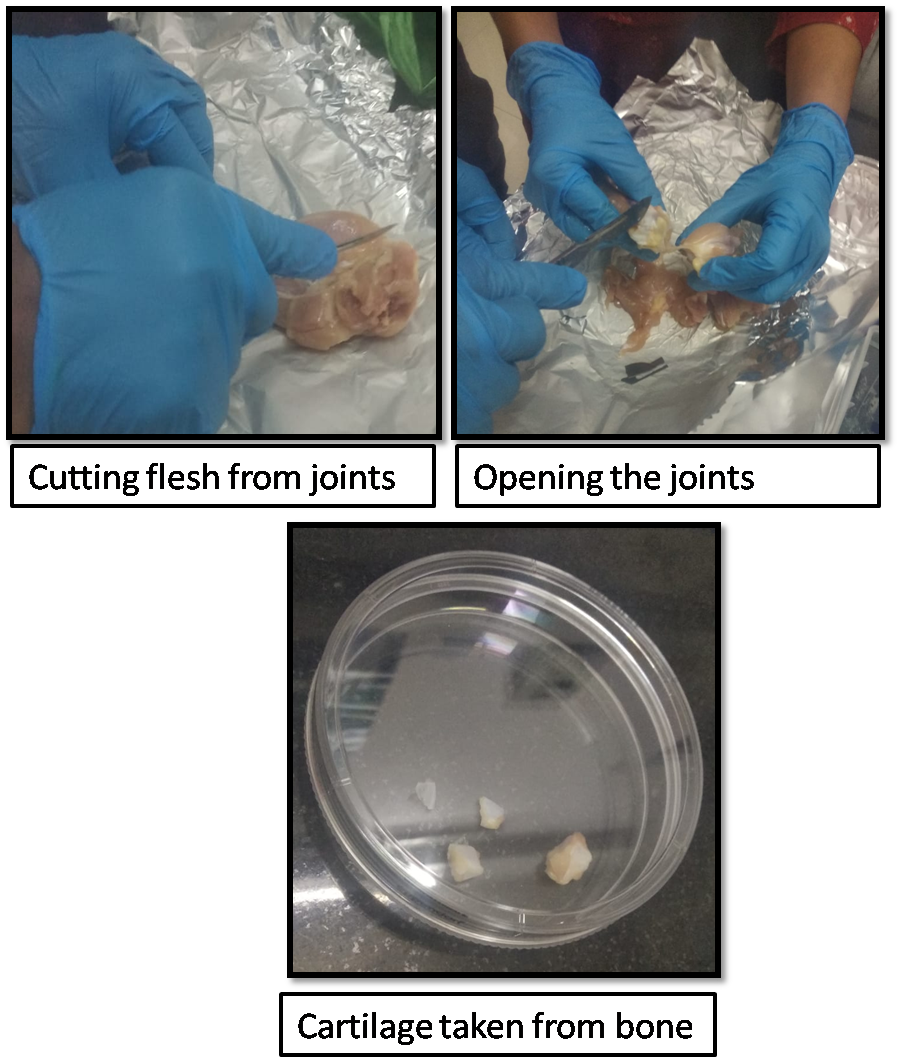
- Place cartilage shavings in tube with PBS.
- Aspirate and add 25 mL fresh PBS to wash.
- Repeat step 11 twice.
- Aspirate PBS and then add 25 mL collagenase solution.
- Incubate in collagenase overnight.
- Vortex cell solution.
- Place cell strainer onto 50 mL tube.
- Pipette cell solution over cell strainer. Try to avoid large cartilage chunks
- Remove the cell strainer and cap tube.
- Spin at 500 g for 15 minutes.
- Prepare agarose solution during spin.
- Prepare 4% (weight/volume) agarose solution (e.g. 4g agarose in 100 mL PBS) in glass bottle.
- Loosen lid and microwave at 50% power at 30-second intervals until dissolved. Swirl gently between each interval. Do not let boil, this will evaporate the water and increase the agarose concentration, which will make the solution difficult to work with
- Spray outside of bottle with ethanol and transfer to biosafety cabinet.
- Spray thermometer with ethanol and dry in biosafety cabinet by gently shaking until ethanol evaporates
- Remove cap from bottle and insert thermometer.
- Let agarose cool to 42°C. Occasionally stirring with thermometer to ensure even cooling.
-
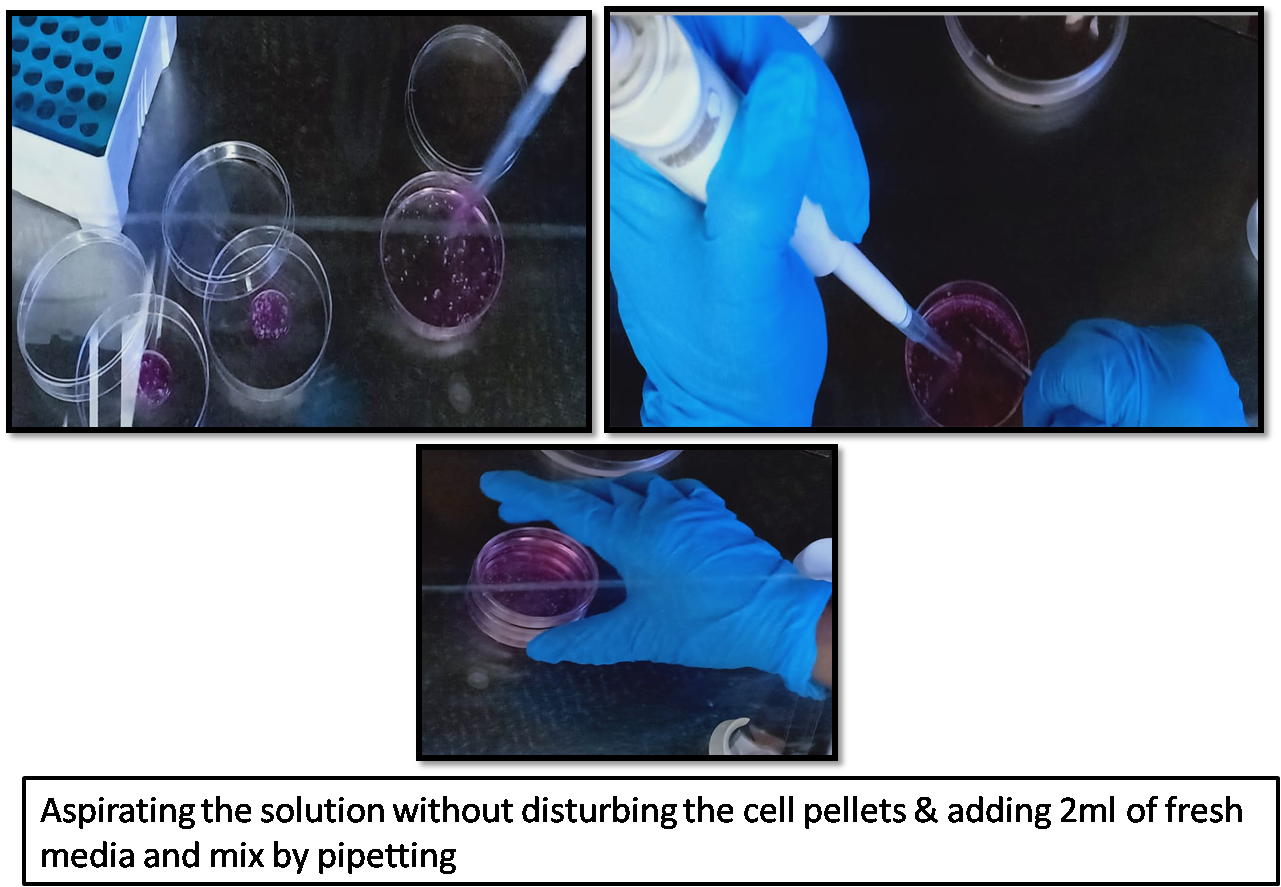
Aspirate solution without disturbing the cell pellets.
- Add 2 mL of fresh media and mix by pipetting.
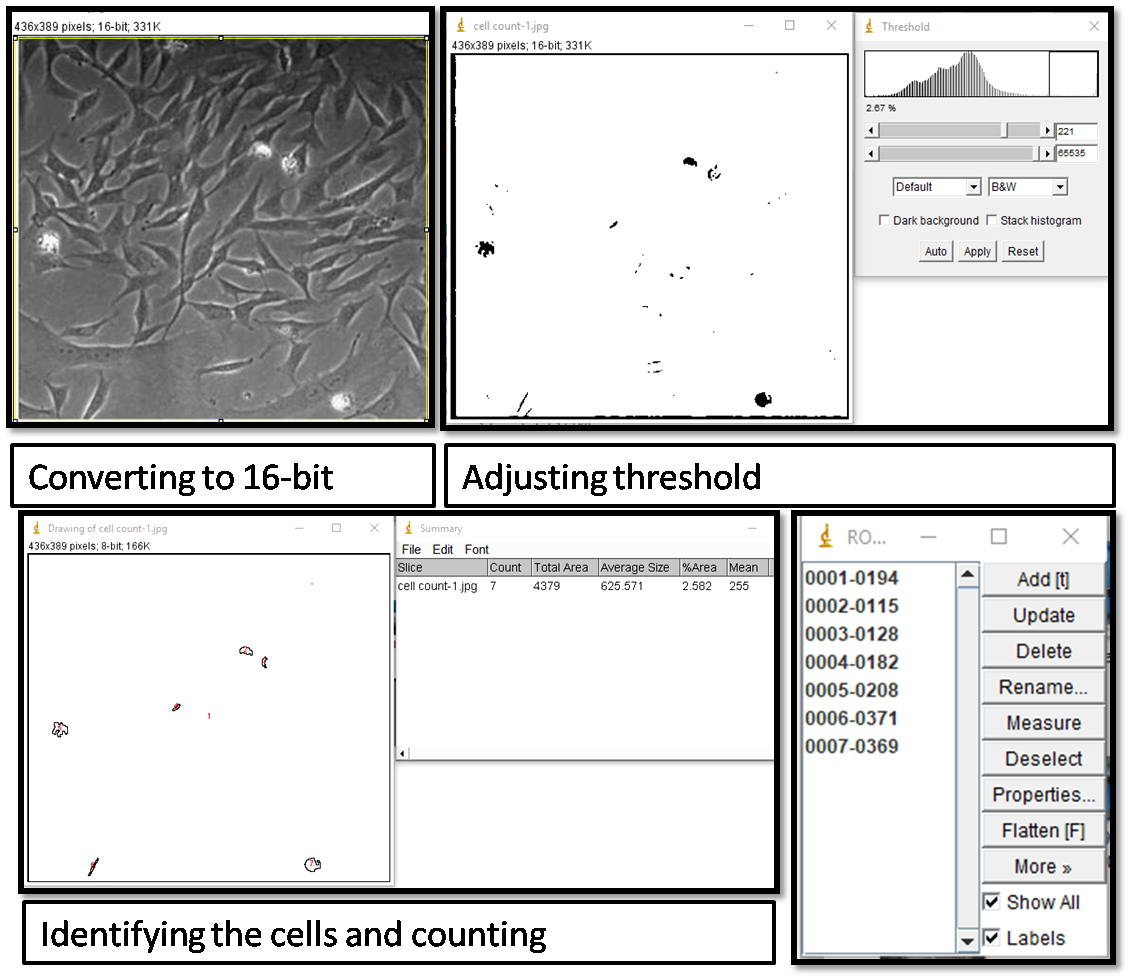
- Cell counting: I used ImageJ software to count the cells.
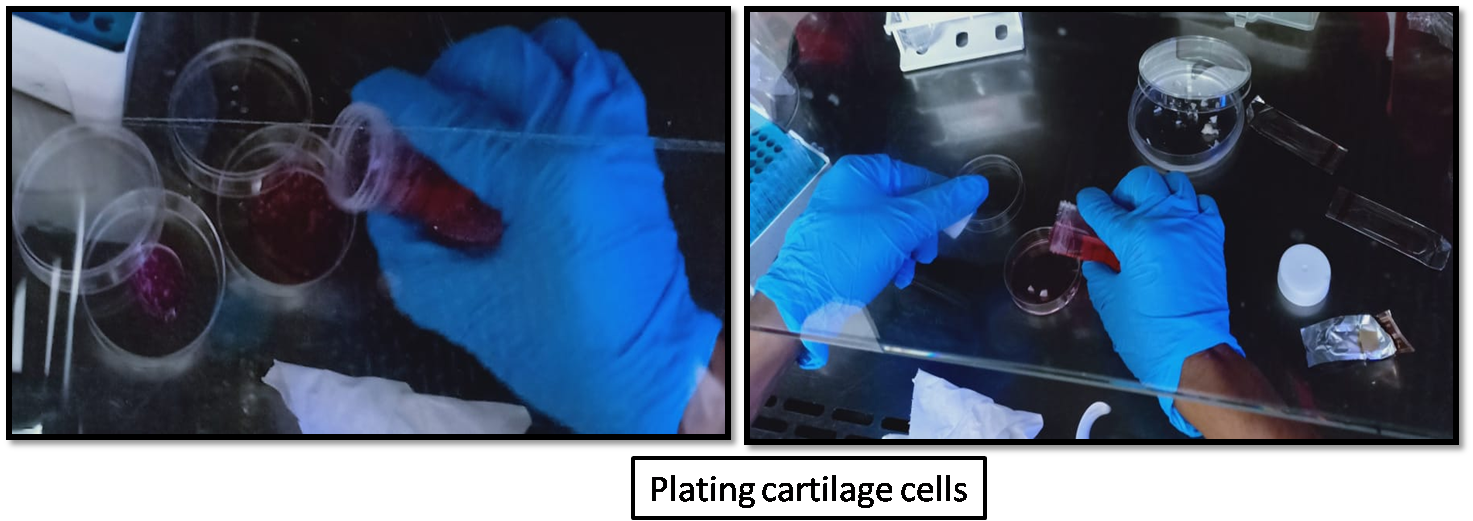
- Combine 2 mL each of cells and 2 mL agarose and mix by pipetting. (Lesson: What’s final concentration of cells in 2% agarose?)
- Pipette 0.5 mL/well cell-agarose solution into 48-well plate.
- Let solidify at room temperature for 15-20 minutes.
- Using the biopsy punch to core out the center of wells and transfer cartilage constructs to fresh 24-well plate containing 2 mL of media.
- Take pictures of 3-4 constructs then do LIVE/DEAD analysis.
- The LIVE/DEAD analysis was not done because of the unavailability of reagents.
- Incubate remaining constructs at 37C/5% CO2 to grow cartilage for 1 week.
- Change media ever 3-4 days by removing old media and adding fresh media.
Changes in color of the media before and after media change.
I noticed that the colour of the media changed from red to orange indicating that the cells are consuming the nutrients, thus decrease in pH. But I did not take pictures of the same.
- Take picture of constructs
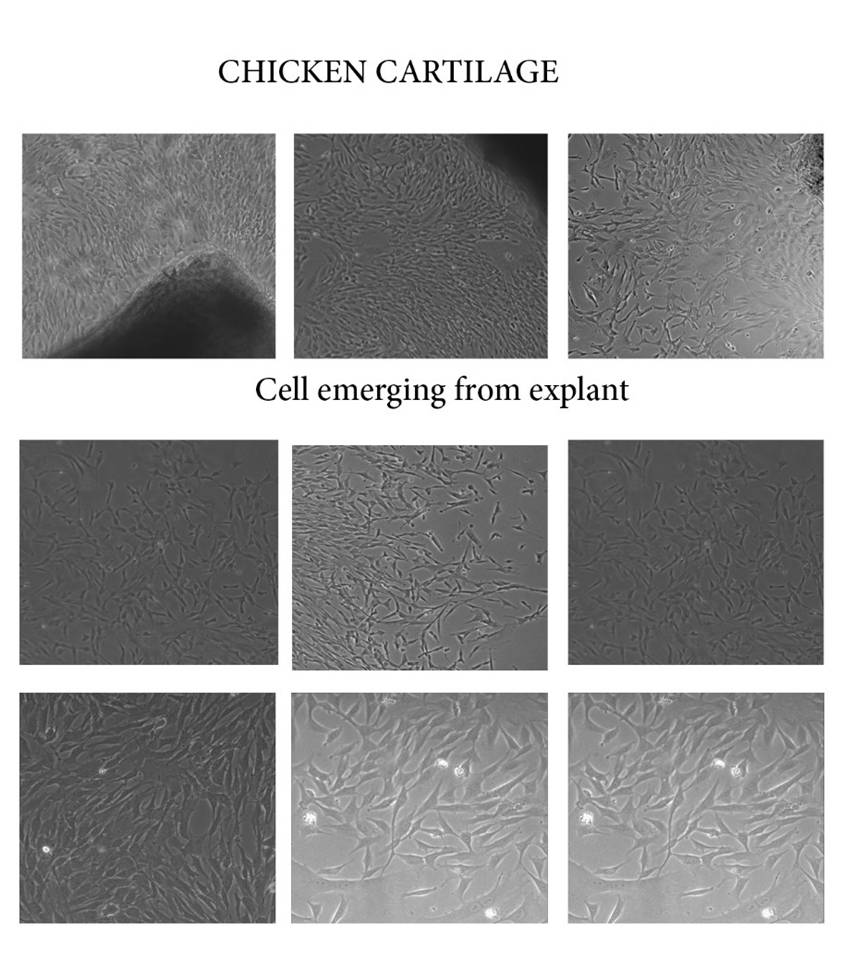
How did the appearance of constructs change during culturing?
The cells started growing slowly over a period of 1 week and the cells gradually turned from conical shape to circular shape.
Go back to home page




